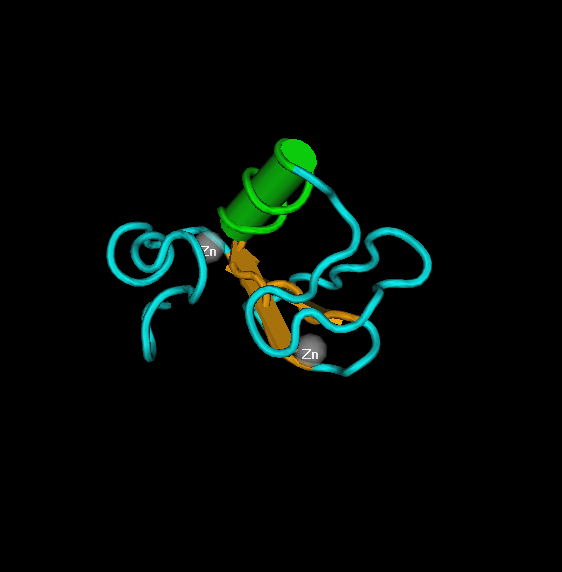
Structure of the the Human Tfiih Mat1 subunit, the N-terminal domain
Primary citation: Gervais, V., Wasielewski, E., Busso,
D., Poterszman, A., Egly, J. M., Thierry, J. C., Kieffer, B.: Solution Structure
of the N-Terminal Domain of the Human Tfiih MAT1 Subunit: New Insights Into
the Ring Finger Family To be Published
http://www.rcsb.org/pdb/cgi/explore.cgi?pdbId=1G25