Comparison of Bacterial RNA Polymerase and Eukaryotic RNA Polymerase
II
RNA Polymerase is an enzyme that is conserved in all
living organisms. Because bacterial, archaeal, and eukaryotic RNAPs are large
enzymes comprising many subunits, they are part of a conserved protein family
called “multisubunit RNAP family (Ebright 2000).” The bacterial RNAP core
enzyme is made up of five subunits (b’, b, aI, aII, and w), with a molecular
mass of approximately 0.35 MDa (Minakhin 2001). Eukaryotic RNAP II consists
of these five conserved subunits and seven additional subunits, with a molecular
mass of about 0.5 MDa. It has been shown that the subunits of the bacterial
RNAP core enzyme and the subunits of eukaryotic RNAP II exhibit sequence,
structural, and functional homology (Minakhin 2001).
The b’ subunit of bacterial RNAP, which is largest one and plays a role
in catalysis, corresponds to the eukaryotic RNAP II subunit RPB1. The bacterial
RNAP b subunit, which is the second largest subunit and also takes part
in catalysis, corresponds to the eukaryotic RNAP II subunit RPB2. The aI
and aII subunits of bacterial RNAP, which have identical sequences but different
locations, with aI interacting with b and aII interacting with b’, correspond
to the RNAP II subunits RPB3 and RPB11, respectively, and play a role in
RNAP assembly and transcriptional regulation (Minakhin 2000). Finally, the
RNAP subunit w corresponds to the RPB6 subunit of RNAP II (Table 1
).
Bacterial RNAP
|
Eukaryotic RNAP II |
b’
|
RPB1
|
b
|
RPB2
|
aI
|
RPB3
|
aII
|
RPB11
|
w
|
RPB6
|
Table 1. Conserved subunits of bacterial RNAP
and eukaryotic RNAP II
Structural similarities between bacterial RNAP and eukaryotic RNAP II
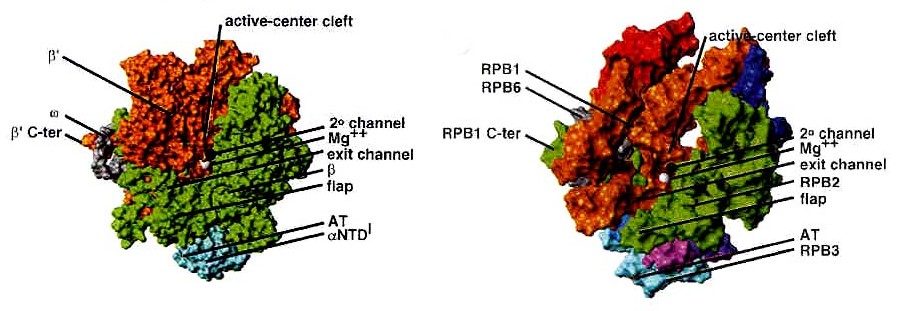
Fig. 1. Structures of Thermus aquaticus core
RNAP enzyme at 3.3 A resolution and yeast RNAP II D4/7 at 3.0 A resolution.
Adopted from Ebright 2000.
Bacterial RNAP is approximately 150 A long, 115 A
tall, and 100 A wide. The X-ray crystal structure of Thermus aquaticus
core RNAP revealed that the enzyme has a shape similar to a crab claw,
with the two arms of the claw defining a wide internal cleft between them
(Zhang 1999). This cleft is about 27 A in diameter, which can accommodate
a double stranded nucleic acid molecule and contains the active center
Mg2+. The b’ subunit makes up one arm of the claw and a portion of the
base of the cleft, while the b subunit makes up the other arm and a portion
of the base of the cleft (Ebright 2000).
Like bacterial RNAP, RNAP II has a shape similar to
a crab claw, with the two arms of the claw forming a deep cleft between
them (Cramer 2000). As Fig. 1 shows, the relative positions of the
conserved subunits in bacterial RNA and eukaryotic RNAP II are the same
(Ebright 2000). Hence, RPB1 forms one arm of the claw and a portion of
the base of the cleft, while RPB2 forms the other arm of the claw and a
portion of the base of the cleft. The nonconserved subunits are
HOME to RNA Pol II in Eukaryotes and Prokaryotes
BACK to Transcription complex stability
NEXT to works cited
