50S subunit
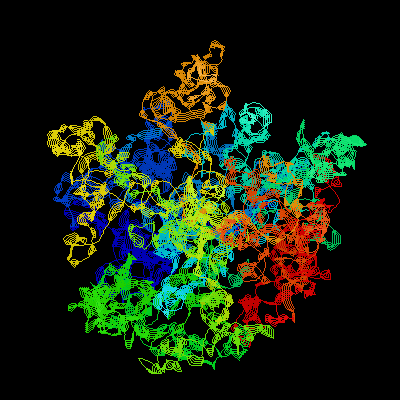
The large subunit of the ribosome is involved in peptide bond formation through peptidyl transferase activity. In addition, GTP binding proteins can interact with the subunit to aid in translational processes such as initiation, elongation, and termination. (Ban et al. 2000) Two rRNA molecules are involved in this process: the 23S and 5S structures, which constitute the backbone of the ribosome complex. 23S rRNA has six interwoven domains that make-up most of the 50S subunit. Portions of domains II, IV, V, and VI extend from the body of the 50S subunit. Some of these extensions form bridges with the 30S subunit. The most important feature of the 23S rRNA is the peptidyl transferase center, the site where peptide bonds are formed.
The role and structure of the 5S rRNA.
The structure of the 23S rRNA.
The ribonuclear proteins involved in the large subunit appear to have a primary function of stabilization of the three-dimensional rRNA structure. In addition, many of the proteins have unique involvement in the initiation, elongation, or termination processes. However, the proteins appear to proliferate around the periphery of the complex, suggesting that they are only involved in increasing the fidelity of mRNA and tRNA binding in translation rather than directly involved in the peptidyl transferase activity and 50S-30S interaction. (Ban et al. 2000)
Within the past five years, 14 of the 34 proteins involved in the large ribosomal subunit have been independently isolated. For more information concerning the role of ribosomal proteins that have been previously isolated from the large subunit can be located here.
In 2000, Ban et al. contributed significantly to our understanding of the remaining proteins by solving the complete structure of the large ribosomal subunit. For a brief summary of the remaining protein structures, please click here.