The Bacterial Ribosome
The bacterial ribosome is a cytoplasmic nucleoprotein particle whose main function is to
serve as the site of mRNA translation and protein synthesis. The ribosome has a mass
of about 2.5 MDa, with RNA accounting for 2/3 of the mass. It consists of two subunits
denoted 30S (small subunit) and 50S (large). When joined, the ribosome has a
sedimentation coefficient of 70S as opposed to 80S due to
tertiary structure. The subunits' shape and arrangement are illustrated
below.
Click on a subunit to learn more.
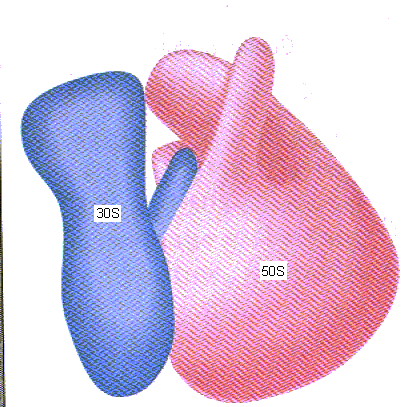
image taken from Weaver RF. Molecular Biology (1999). 602.
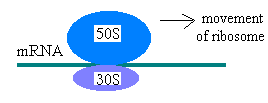
During protein synthesis a ribosome moves along an mRNA molecule, reading the codon and
adding the correct amino acid (from the corresponding aminoacyl tRNA) to the growing protein.
When a stop codon is reached,translation ceases, and the mRNA and protein are released.
The two subunits assemble around a mRNA to be translated as such:
Electron microscopy was the main technique used to discover the structure of the bacterial ribosome. The
first electron micrographs revealed that ribosome consists of two subunits, one large and one
small. James Lake in the 1970s was able to obtain electron micrographs a higher resolution, which
enabled him to determine the shape of each subunit (50S and 30S) and how the subunits fit together
(70S). Also in 1970, E. Kaldschmidt and H.F. Whittmann used two-dimensional gel electrophoresis to
obtain nearly complete resolution of both the subunits and the ribosomal proteins. Kaldschmidt and
Whittmann were able to identify proteins S1-S21 for the 30S ribosomal subunit and proteins L1-L33 for the
50S subunit. In 1995, Joachim Frank worked with several other scientists to use cryoelectron microscopy
to determine the structure in more detail. Scientist Masayasu Nomura and others were able to determine
the approximate locations of proteins within each subunit.


