S2
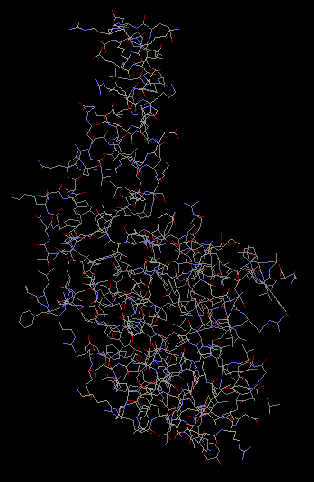
Protein S2 has an alpha-hairpin located within it. This was
determined during the crystallization of the 30S
subunit. The S2 protein is beloved to be involved in
the mediation of interaction between the head of the
ribosome and the protein (Wimberly et al 2000).
S3
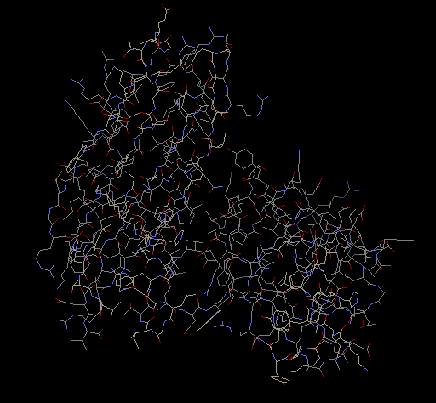
The exact structure of protein S3 is not yet known.
From the crystallization of 30S subunit, it was found
that there is a beta-pleated sheet, along which alpha
helices are stacked. There are two globular domains,
alpha and beta. One of the domains is isolated from
the RNA. Protein S3 is involved in a cluster with
proteins S10 and S14. Hydrophobic interactions
stabilize the cluster, which then helps stabilize the
structure of the ribosome (Wimberly et al 2000).
S10
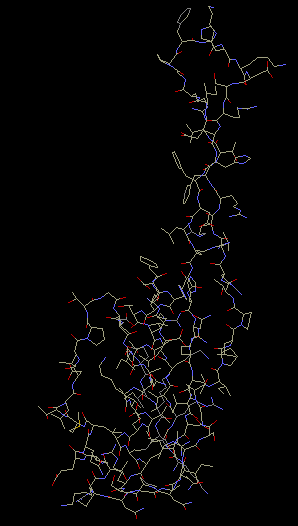
It has been found through crystallization of the 30S
subunits that S10 contains alpha helices packed against a
beta-pleated sheet. It also contains either a long
hairpin or loop, which either could be used to
interact with 16S rRNA. Protein S10 is involved in
a cluster with proteins S3 and S14. Hydrophobic
interactions stabilize the cluster, which then helps
stabilize the structure of the ribosome (Wimberly et
al 2000).
S11
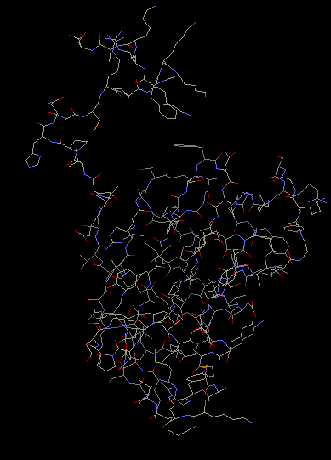
S11 has a beta sheet with alpha helices
stacked around it. The Beta sheet is located near the
minor groove of 16S rRNA. S11 is packed flatly
against the groove. Also, S8 has an extended tail
from either the carboxyl or amino end of the protein.
The extension allows the protein to interact with 16S
rRNA and make contact with the rRNA (Wimberly et al
2000).
Return to all proteins
Return to index



