S5
S5 has two definite domains of alpha helices
and beta-pleated sheets. Its molecular mass is 17500.
There are two distinct sections of S5 that may be
interaction sites for 16S rRNA. This was found by
mapping various mutations that occurred in S5 to these
distinct sections of the protein. The mutations in
the S5 protein cause several different phenotypes to
occur. These include ribosome ambiguity or ram,
reversion from streptomycin dependence and resistance
to spectinomycin. The mutations suggest that protein
S5 is involved in translocation and translational
fidelity (Ramakrishnan and White 1992).
S6
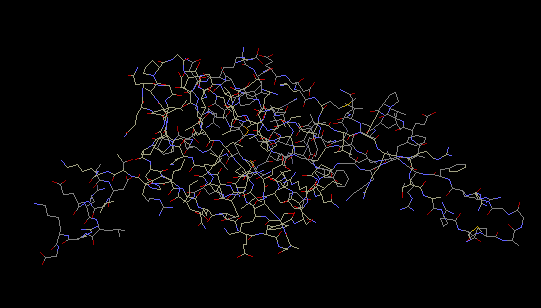
The crystal structure of protein S6 shows that the S6
protein consists of only 101 amino acid residues. It
is composed of one “four-stranded anti-parallel
beta-sheet with two alpha helices packed on one side,”
(Lindahl et al 1994). RNA interacts with the edge of
the beta sheet (Wimberly 2000).
S9
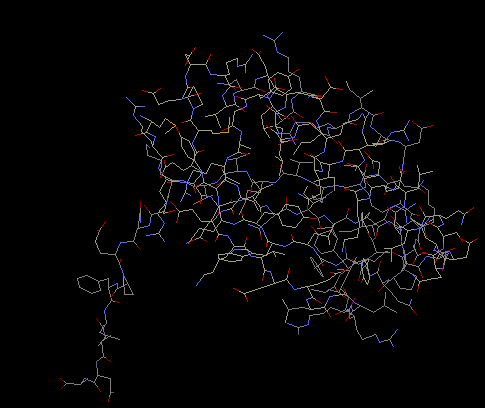
Protein S9 has one main globular
domain, and one long extension, either one the carboy-
end or the amino-end, which protrudes away from the
rest of the protein like a long tail. The extension
is believed to be used to interact and make contact
with 16S rRNA. The extension is narrow which allows
for the close contact with the rRNA. Also, along the
extension are basic amino acid residues, which
neutralize the rRNA backbone’s charge repulsion
(Wimberly et al 2000).
S12
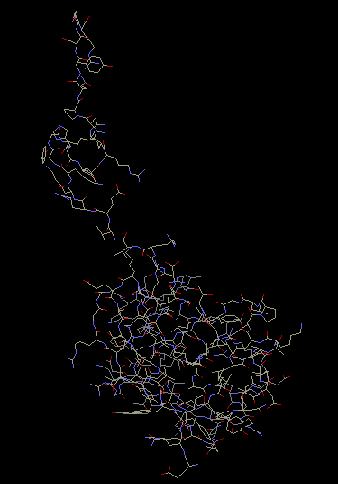
S12 consists of globular domains, and
an extended carboxyl or amino end tail. This allows
the protein to make contact with 16S RNA. The
globular domain is located on the interface side of
the 16S rRNA. The tail weaves through the body of 16S
rRNA and then folds into a small alpha helix. The
alpha helix can then interact and make contact with
proteins S8 and S17 (Wimberly et al 2000).
Return to all proteins
Return to index



