S1
There is not much information yet known about protein S1.
Scientists do know that the RNA binding
domain is at the C-terminal end of the protein. This
end may be necessary for autoregulation of the
ribosome (Boni et al 2000). Using mobility assay,
scientists discovered that S1 worked with elongation
factor EF-TU and protein SmpB, to regulate the tmRNA
binding to ribosomes and to regulate the formation of
free tmRNA complexes (Wower et al 2000).
S4
The structure of S4 was determined by multidimensional heteronucleaar nuclear magnetic
resonance spectroscopy. S4 is made up of two globular domains; domain one consists of four alpha
helices, and domain two consists of one five stranded antiparallel beta-sheet with three alpha helices
packed on one side.” Domain two is located within domain one (Markus et al 1998). The crystal
structure of protein shows that S4 has an extended amino terminal chain. The
extension enables proteins to interact with 16S rRNA. S4 Delta 41, which consists of nucleotides 43-200
in Bacillus steearothermophilus, binds specifically to 16S rRNA and the alpha operon mRNA’s pseudoknot
(Markus et al 1998). S4 binds to the 5’ domain of the 16S rRNA. It is important in the assembly of the
body of the
ribosome.
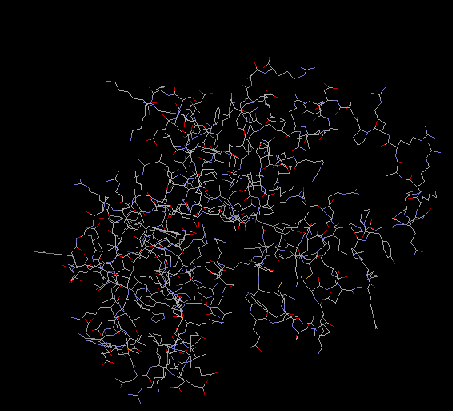
S7
From a two-wavelength diffraction experiment that used
LIII edge of mercury, the crystal structure at 1.9 A
of Thermus thermophilus was determined. S7 is made up
of a beta hairpin extended between helices three and
four in a cluster of six alpha helices. There are
believed to be several RNA-binding sites along its
surface. Helices one, four and six, and the
beta-hairpin make a positively charged curved surface
which is perfect for binding double-stranded RNA
(Wimberly, White, and Ramakrishnan, 1997). At 2.5A
resolution, an experiment was done using
multiwavelength diffraction with
selenomethionyl-substituted proteins. The
crystallization revealed that there was a hydrophobic
core with beta-sheets extending from the core. The
core is made up of several helix-turn-helix motifs.
Basic and aromatic amino acid residues are located on
one side of the S7 protein, which is where the
RNA-binding sites are believed to be located. S7 is
located in the head of the 30S subunit. There it
initiates assembly of the head of the 30S subunit. S7
is involved in the cross-linking of tRNA. (Hosaka et
al 1997). S7 is also required for the folding of the
3’ domain of 16S rRNA and it binds to its own mRNA to
regulate its own synthesis (Wimberly, White, and
Ramakrishnan, 1997).
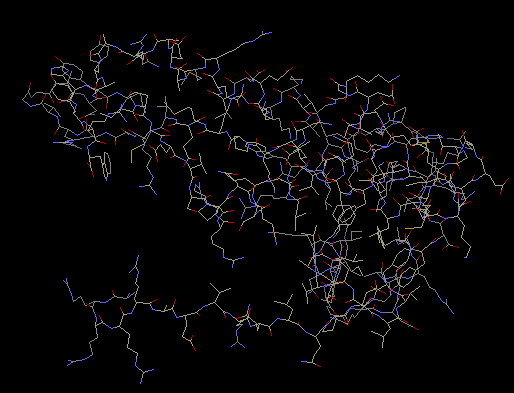
S8
Using NMR spectroscopy, the structure of protein S8 in
Escherichia coli was determined. Protein S8 consists
of two domains of helical segments, two RNA-binding
sites, one of which is located from G588 to G604
nucleotides and the other from C634 to C651
nucleotides. There is also a hydrophobic core, which
contains nine amino acid residues and is possibly
associated with protein S5. Within the core is a
triple base pair, nucleotides A595 x (A596 x U644).
S8 plays a role in translation regulation of ribosomal
proteins. The protein S8 also interacts with spc
operon mRNA. Through this interaction, S8 is able to
play a key role in the regulation of translation for
several other ribosomal proteins (Kalurachchi et al
1997). S8 is an important RNA-binding component. It
independently binds to 16S rRNA. In Escherichia coli,
S8 is able to bind to its own mRNA and then regulate
translation by acting as a repressor (Nevskaya et all
1998). S8 is required for the proper folding of the
central domain of 16S rRNA. S8 also binds to mRNA
enabling it to control the synthesis of other
ribosomal proteins (Davies, Ramakrishnan, and White,
1996).
Return to all proteins
Return to index


