L2
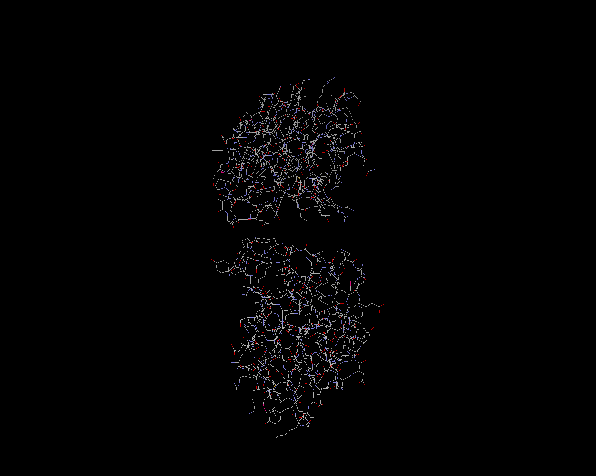
The largest ribosomal protein, L2 also has a dual domain structure with each domain containing approximately 70 amino acids. L2 is involved in the binding and assembly of the 23S rRNA, and is also thought to be involved in peptidyl transferase activity. The region in between the two domains contains two arginine residues (Arg86 and Arg155), which served as a gate and aid in the binding of the rRNA. The structure shown above was determined via X-ray crystallography and the selenomethionyl MAD method using isolated protein from Bacillus stearothermophilus. (Nakagawa et al. 1999)
It is useful to note that a number of proteins, like L1 and L2, have two domains which contain almost identical sequences of amino acids. Nakagawa has suggested that these multiple domains probably stem from ancient ancestral proteins which contained only one domain. The evolutionary impetus appears to be one of fidelity, in which binding of rRNA was much tighter using a clamp technique of two domains rather than just attaching using two or three amino acid residues. The study of these proteins can shed some light on the evolutionary impetus behind the formation of multiple domains and attachment of muliple protein residues.