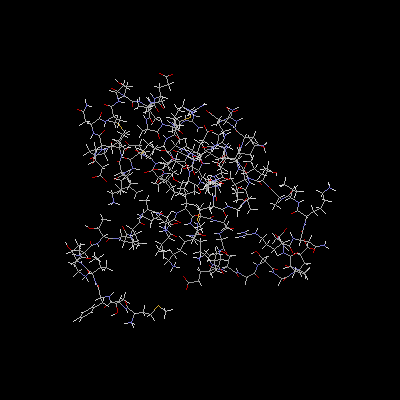
L7, L10, L11, L12
The L7/L12 stalk is a complex of proteins involved in the elongation process of protein translation; specifically, L7, L10, L11, and L12 are involved in the complex formation. L7 and L12 exist as a dimer, as the two proteins are identical in amino acid sequence and differ only in the acetylation of the L7 residue. In addition, the L12 protein will associate with partial L12 sequences to form a "hetero-tetrameric complex", according to Wahl et al. (2000)
L12
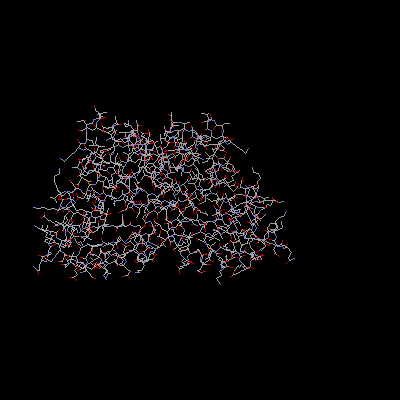
In addition, the L10 and L11 proteins are involved in the L7/L12 stalk. The structure of the L10 protein has not yet been isolated, but the protein is projected to be involved in support of the L11 protein (Ban et al. 2000). The C-terminus of L11 binds tightly to the 23S rRNA, while the N-terminus is involved in further interactions that can be reversed. Thus, L11 is projected as a switch that allows for elongation of the translated protein to take place. One experiment in support this theory involves the thiostrepton antibiotics, which will bind L11 and prevent elongation from occuring. (Wimberly et al. 1999)
L11