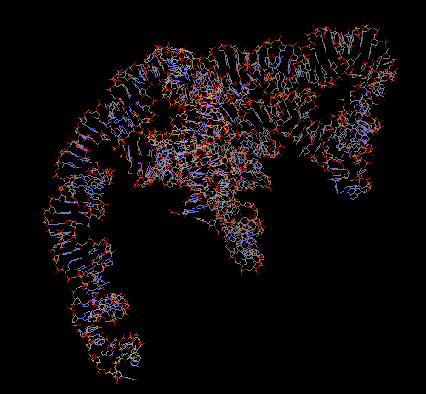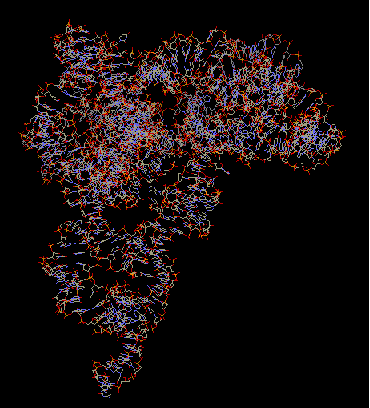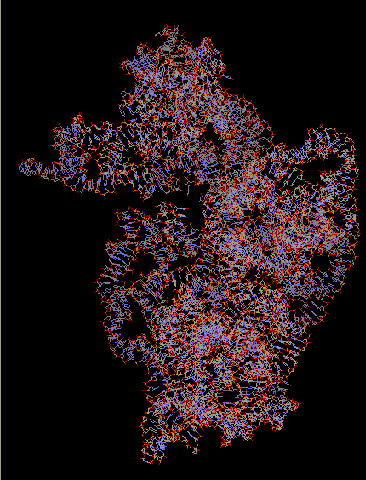
16S rRNA makes up the bulk of the 30S subunit. It is important for subunit association and translational accuracy. It consists of 1542 bases and contains the substrate binding A-, P-, and E- sites. The P- site is occupied by peptidyl-tRNA and is located in the major groove in an upper portion of the rRNA. The A- site is the attachment site for an incoming aminoacyl-tRNA, and is wide and shallow, which gives it a lower affinity for tRNA so it may relocate to the P- site. The E- site, occupied by deacylated tRNAs when they exit, is associated with ribosomal proteins more than the A- or P- site, perhaps facilitating the dissociation of the codon-anticodon pair during translation.
The primary structure of 16S rRNA is highly conserved. Its secondary structure is reminiscent if the clover shape of tRNA-small double helical regions are punctuated by short single stranded regions. The tertiary structure is the general shape of the whole subunit.
The arrangement of the 16S rRNA creates a 5' domain, central domain, 3' major domain, and a 3' minor domain.