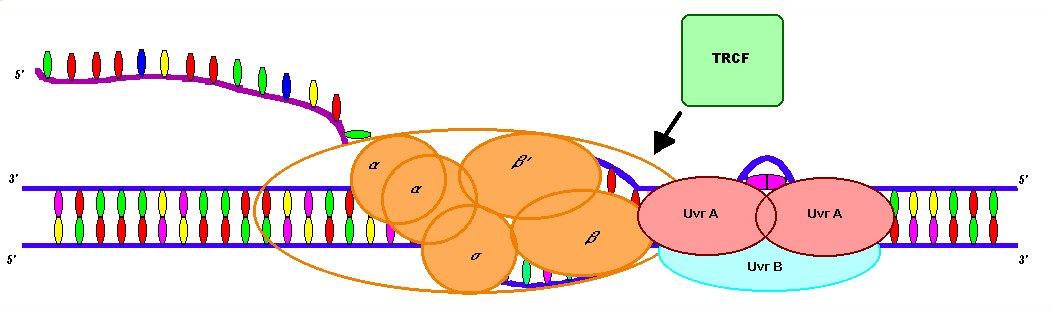
The transcription-repair coupling factor (TRCF) is involved in linking transcription to DNA repair. The mfd (mutation frequency decline) gene encodes TRCF. TRCF is an ATPase that is approximately 130 kilodaltons in size, and is made up of helicase and leucine zipper motifs (Selby and Sancar, 1993). TRCF enhances the rate of repair of lesions to the transcribed strand of DNA (Lin, Kovalsky, and Grossman, 1998).
A suggested mechanism for TRCF indicates that when RNA polymerase is stalled at a lesion, the repair complex, UvrA2 B, is already bound near the damage site. Transcription generates positive and negative supercoiling around the lesion, which leads to the formation of a looped DNA domain around the lesion. TRCF then binds between the UvrA2B complex and the RNA polymerase. TRCF causes the release of RNA polymerase, which provides more room for UvrA 2B to efficiently repair the damaged DNA by allowing flexibilty around the damage site that was prevented by the RNAP complex. This mechanism suggests that, in addition to TRCF, the supercoiling generated by transcription increases the rate of repair because it allows efficient recognition of the lesion (Mellon and Champe, 1996). Using TRCF covalently bound in an affinity column, it has been shown that TRCF can bind to UvrA at the same spot that UvrB binds. This facilitates the dissociation of UvrA from the UvrA2B complex (Selby and Sancar, 1993).
Another proposed TRCF mechanism indicates a slightly different mode of repair. As before, TRCF recognizes and releases the stalled RNA polymerase. However, the UvrA2B complex has not yet bound to the DNA. Instead, TRCF recruits UvrA 2B to the damage site once the RNA polymerase has been released. The UvrA2B complex can then begin its repair activity (Buratowski, 1993).
Studies using mfd knockout experimental
strains have indicated that TRCF is not necessarily required for transcription-coupled
repair. The complete role of TRCF in a cell remains unclear (Lin, Kovalsky,
and Grossman, 1998).